Treatment Overview
Vagus Nerve Stimulation
The immune system typically uses inflammation to help your body fight infection and recover from injury. However, too much inflammation leaves the immune system unbalanced, causing autoimmune diseases like RA.
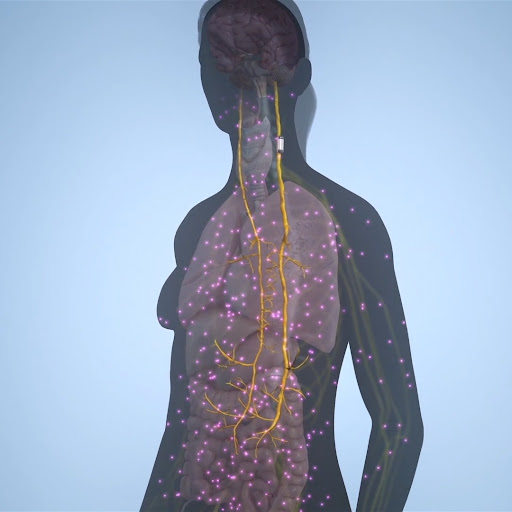
In healthy individuals, inflammation is naturally regulated by an internal pathway- the inflammatory reflex. This inflammatory reflex is controlled by the vagus nerve, which helps to manage the body’s immune system by balancing inflammation.
Vagus nerve stimulation uses an implanted device to apply a small amount of electricity to the vagus nerve, which may decrease the kind of inflammation that is present in people with Rheumatoid Arthritis (RA).
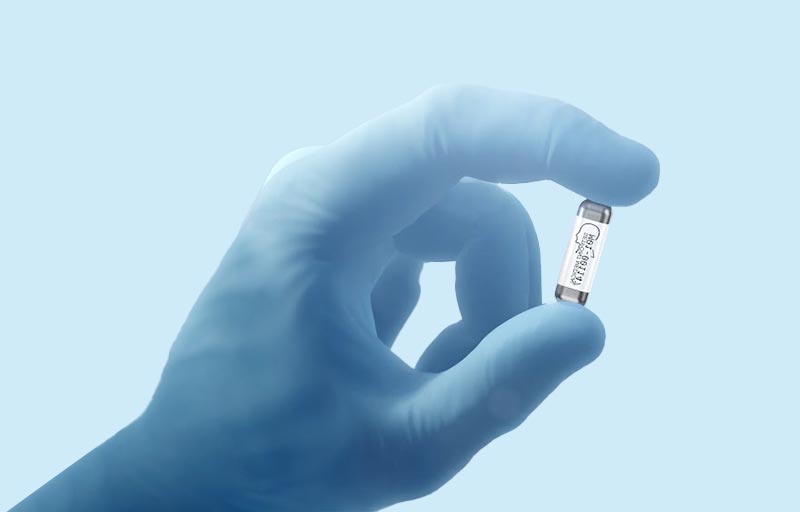
MicroRegulator Implant
Vagus nerve stimulation devices are currently used for the treatment of epilepsy and depression. Because the vagus nerve controls various body functions, including the inflammatory reflex, it has the potential to treat autoimmune diseases, such as Rheumatoid Arthritis (RA). MicroRegulator is a new device designed specifically to deliver vagus nerve stimulation for applications like RA.
MicroRegulator is an investigational device about the size of a multivitamin capsule, designed to provide electrical stimulation to the vagus nerve specifically to treat RA.
MicroRegulator does not administer any drug into the body, but rather, the device applies small electrical impulses to the vagus nerve for about 1 minute a day to activate the body’s natural immune response, which may treat RA.
Physicians use an iPad to program each patient’s MicroRegulator to turn on and deliver the therapy automatically every day so that patients do not need to remember to turn on the device.

Implant Procedure
The MicroRegulator is placed on the left vagus nerve through a small, inch-sized incision on the left side of the neck. The 60-90 minute implantation procedure is performed by an experienced surgeon while the patient is under general anesthesia. The patient typically returns home a few hours after the procedure, and can resume daily living activities the following day.
The implantation procedure is performed by a surgeon with experience in vagus nerve stimulation implant procedures. Before the procedure, your study surgeon will meet with you for pre-surgical clearance. During that time they will review the procedure and can answer additional questions you may have.
Thank you for your interest in the RESET-RA Study. Enrollment in the study has concluded.
The next phase of the study is to follow the current participants to determine the outcomes of the study. While we await the results, we have partnered with the Global Healthy Living Foundation (GHLF) to keep you up to date on the study and provide additional educational resources for management of RA.
Please visit CreakyJoints.org/reset-ra for a community of support, free resources, and tools to help you live better with RA.
As the parent organization of CreakyJoints®, a worldwide digital community serving millions of arthritis patients and caregivers, GHLF provides free education, support, advocacy, and patient-centered research in both English and Spanish.