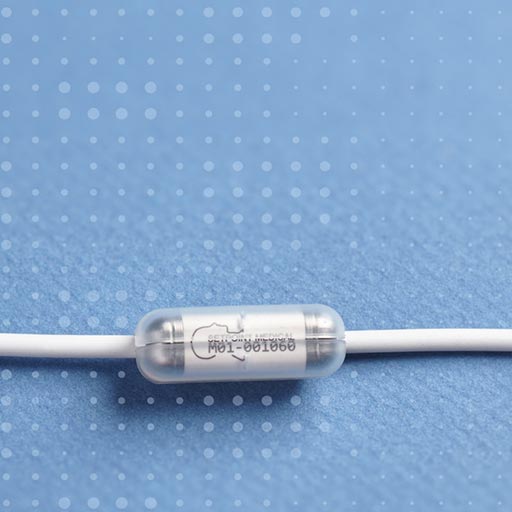
About Reset-RA Study
What is RESET-RA?
The research in the RESET-RA study will help determine if an investigational vagus nerve stimulation device is safe and can improve Rheumatoid Arthritis (RA) in patients who continue to experience symptoms even after having tried multiple medications.
What is a clinical research study?
Discovering new effective therapies for conditions like RA depends on clinical research. As participants in this study, the patients are partners in research and play an important role in advancing the treatment choices for RA. Participants will help researchers evaluate if this novel therapy is safe and effective, giving them the ability to try the new potential treatment option while it is under investigation.
Who can participate in the study?
Study participants must meet the following initial requirements in order to be further evaluated for participation:
- Be 22-75 years of age
- Have been diagnosed with RA as an adult
- Have active moderate to severe RA
- Are currently taking a conventional synthetic DMARD. Click here to see a list of common csDMARDs
- Have received at least one biologic DMARD (biologics) for RA that was either not effective or was not tolerable. Click here to see a list of common biologics
- Are willing to undergo an investigational vagus nerve stimulation implant procedure
Who is behind the RESET-RA study?
The RESET-RA Study is sponsored by SetPoint Medical Inc., a privately-held clinical-stage healthcare company dedicated to patients with chronic autoimmune diseases. As part of the company’s pioneering research into a new treatment paradigm – which uses electrical impulses that are designed to activate the body’s innate “Inflammatory Reflex,” for rheumatoid arthritis, Crohn’s disease and other chronic autoimmune diseases – SetPoint is conducting the RESET-RA study based guidelines set forth by the FDA.